Recently, I read a Forbes Article entitled, “Why CRISPR Is An Attractive Approach To Treat Sickle Cell Disease” it states “Using CRISPR to edit a short segment of DNA in the cells, they activated a genetic switch that effectively raised the levels of fetal hemoglobin in red blood cells, turning the cells healthy.”
1. Can you briefly explain CRISPR and discuss whether the red blood cells continue to produce fetal hemoglobin or if normal hemoglobin would be produced on its own as a result of this treatment. Is this type of treatment potentially a long-term solution or cure?
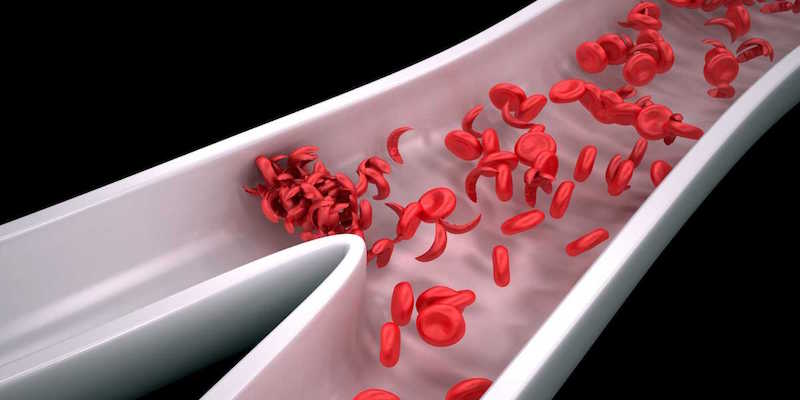 CRISPR is a powerful new way to edit DNA precisely. CRISPR allows scientists to perform genomic surgery whereby they can remove or introduce new genes into existing DNA. For example, scientists can use this technology to simply snip out genes, or cut out genes that do not work and replace them with new, more effective ones. The CRISPR enzymes were first discovered in bacteria which use them to protect themselves from infection. Scientists have discovered that the bacterial system can be harnessed to make precise changes to the DNA of humans, other animals and plants. The blood stem cells in the St. Jude study would continue to produce fetal hemoglobin on their own as a result of this procedure. This potential treatment is in theory a long-term solution or cure but much work remains to be done before the work can reach the clinic and be shown to be safe and effective.
CRISPR is a powerful new way to edit DNA precisely. CRISPR allows scientists to perform genomic surgery whereby they can remove or introduce new genes into existing DNA. For example, scientists can use this technology to simply snip out genes, or cut out genes that do not work and replace them with new, more effective ones. The CRISPR enzymes were first discovered in bacteria which use them to protect themselves from infection. Scientists have discovered that the bacterial system can be harnessed to make precise changes to the DNA of humans, other animals and plants. The blood stem cells in the St. Jude study would continue to produce fetal hemoglobin on their own as a result of this procedure. This potential treatment is in theory a long-term solution or cure but much work remains to be done before the work can reach the clinic and be shown to be safe and effective.
2. What age group will CRISPR target?
In theory, the CRISPR technology could be used to edit the blood stem cells from patients of different ages. In practice, it would be better to use this approach as early as possible since the health effects of sickle cell accumulate over a lifetime due to the result of impaired oxygen supply to the tissues and organs of the body. Sickle cell patients have to manage their disease and this becomes more challenging as they move into adulthood.
3. Is their any potential that research similar to CRISPR can be used to prevent babies in the womb from forming sickle cell?
The ethics of using genetic modifications in human embryos are a topic of much discussion since there are many concerns with this approach. Currently most countries are not pursuing this type of research due to ethical considerations although there have been a few reports of editing genes in human embryos that did not result in live births (more information is available here http://www.nature.com/news/second-chinese-team-reports-gene-editing-in-human-embryos-1.19718). There is potential but there is a lot of work to be done to ensure that this type of approach is both safe and ethical.
4. The article states that the clinical trial for CRISPR will start in June 2017 at the National Institute of Health. What are doctors and researchers looking to see to consider the trial successful?
Currently no clinical trials are in progress for CRISPR and sickle cell disease. Researchers at the Dana-Farber/Boston Children’s Cancer and Blood Disorders Center did announce recently that they may start to enroll patients on a trial next year (https://www.statnews.com/2016/09/07/sickle-cell-gene-therapy/). Other trials may follow for different types of gene editing approaches. The trial mentioned in the FORBES article is for an immunotherapy treatment for cancer that uses CRISPR in work led by the University of Philadelphia (http://www.nature.com/news/first-crispr-clinical-trial-gets-green-light-from-us-panel-1.20137). Clinical trials will have to show that the therapies are safe, effective and have no longer term side effects.
5. How soon after the first clinical trial could CRISPR become available to the public?
This is a very hard question to answer. Clinical trials take many years to complete and it is very important to monitor for potential long-term side effects of any new treatment. For a therapy to reach the public it would have to proceed through a rigorous clinical trial and be approved by the national regulatory agencies, for example the FDA in the United States.
6. Is CRISPR also being used as a potential treatment for other genetic disorders besides Sickle Cell Disorder?
Yes indeed. Work is in progress for other diseases that arise from changes to single genes or a few genes, for example Duchenne’s muscular dystrophy or cystic fibrosis. Scientists are also looking at ways to use CRISPR for gene editing to treat cancer (see last sentence in point 4) and as a way to increase resistance to infection by the HIV virus. Again a lot of work is at the stage before clinical trials begin in humans but some of these are expected to start in the near future.
7. Are researchers/doctors looking for more patients to conduct this trial or other sickle cell related clinical trials?
Currently no therapeutic clinical trials that use CRISPR to treat sickle cell disease are recruiting patients as mentioned in 4 above. One may start enrolling next year. Patients are being recruited for other sickle cell clinical trials and there is a lot of research underway for the disease.
8. Lastly, where can people go to learn more about new Sickle Cell treatment and research?
Further information about clinical trials underway at St. Jude is available here https://www.stjude.org/research/clinical-trials/sickle-cell-disease-clinical-trials.html
Learn more about St Jude and Sickle Cell Disease here: https://www.stjude.org/research/initiatives/sickle-cell-awareness.html

References:
St. Jude Children’s Research Hospital. Living with sickle cell disease: Shaniya’s story. Online Video clip. YouTube. Published on Feb 29, 2016. Retrieved September 20, 2016 from https://youtu.be/bEYqP8iZ8TE
Follow the #30forsicklecell hashtag (on facebook and Twitter):

[notification type=”notification_info” ]Image Source.[/notification]